
ACT-EARLY Locations

If you carry an inherited genetic variant for a rare genetic condition called variant transthyretin amyloidosis (ATTRv), also known as hereditary ATTR, you may be eligible to participate in a study evaluating an investigational approach to help prevent the onset of this disease.
This study is designed to evaluate whether acoramidis can prevent or delay the onset of disease in individuals with hereditary transthyretin amyloidosis (ATTRv) who have not yet developed symptoms.
This study is being conducted in individuals who carry a TTR genetic variant and who have not yet developed symptoms of, or been diagnosed with ATTRv. Participants will be randomly assigned (by chance) to receive either the investigational oral drug acoramidis or a placebo (an inactive substance), with a 50% likelihood for each group.

Test whether acoramidis can delay the onset of or prevent the development of ATTRv disease in individuals who carry a TTR genetic variant and who have not yet gotten sick from or been diagnosed with ATTRv disease

Understand how early treatment, before ATTRv disease has been diagnosed, can impact and possibly prevent the onset of ATTRv

Identify early signs of ATTRv disease through routine clinic visits that will include physical exams, imaging tests of your heart, nerve testing, and blood tests designed to screen for ATTRv
You may be eligible for this study if you are between 18 and 75 years old, have inherited a TTR genetic variant that puts you at risk of developing ATTRv amyloid disease, and are within 10 years or older of the typical age when symptoms of ATTRv amyloid disease are expected to develop.
Use the interactive map to explore participating ACT-EARLY study locations across the United States and internationally.
For more information go to clinicaltrials.gov
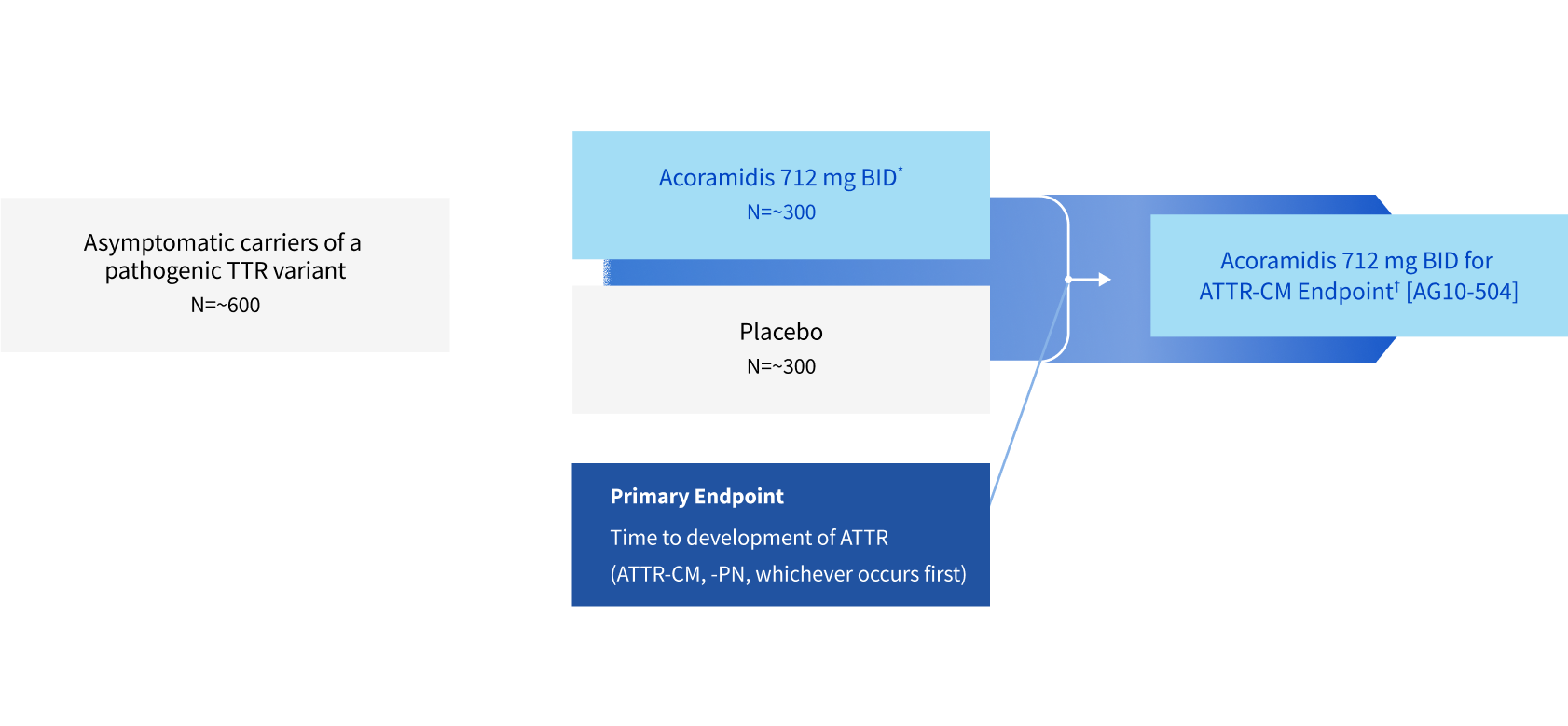
*
Acoramidis 712 mg is the content of the moiety equivalent to the 800 mg acoramidis HCL dose used in the pivotal phase 3 ATTRibute-CM trial.
†
Participants newly diagnosed with ATTR-PN should seek therapy with standard of care outside of this trial.
Screening – Health and genetic history evaluation
Study Treatment Period – Acoramidis or placebo taken orally twice daily
Follow-Up – Routine monitoring about every 6 months including physical exams, imaging of your heart, nerve testing, and blood tests
Start date (estimated): 2025-05
Enrollment ongoing: Explore study locations
Primary completion (estimated): 2031-10
Hereditary transthyretin amyloidosis (ATTRv) is a rare, inherited, disease that can get worse over time and can lead to death if untreated.

Amyloidosis is a group of diseases caused when misfolded proteins, called amyloid, build up and form fibrils that deposit in the body's organs and tissues, affecting their ability to function.
Hereditary transthyretin amyloidosis (ATTRv) is a type of amyloidosis that is caused by a change (also referred to as a mutation or variant) in a gene called transthyretin, which provides the instructions for the transthyretin (TTR) protein.
Normally, the TTR protein circulates in your blood in groups of four units. However, when you have an inherited TTR gene variant, the resulting TTR protein can become unstable and fall apart into pieces that form amyloid. This amyloid then gets deposited in different organs and tissues around the body, most commonly in the heart and around the nerves.1
Over time, this can lead to: